












Biosampling Basics
Purpose: Collecting large quantities of fish can have an undeniable effect on the populations strength and stability. This makes it vital for fishery management to have an accurate understanding of each species’ growth and maturity. Currently, relatively little is known about the growth and maturity of commonly caught coral reef species within Hawaiian waters. This is where biologists, like us, come into play. We are responsible for the collection, analysis and reporting of the life history traits that are specific to these species. Examples of life history information would be the investments fish make in growth and reproduction, the size and age at which they mature, and the maximum age and sizes. The ongoing purpose of this study is to fill these data gaps within Hawaiian populations of reef fish. These life history parameters can then be used to improve stock assessment models to more accurately assess local populations and provide managers with more reliable information to manage our resources sustainably.
The Process: In order to conduct this study, we initially conduct a routine check of local fish vendors to get a monthly estimate on all the different reef species being caught and sold here in Hawai’i. However, we only do in-depth life history studies on around 9 species. In order to do this, we first start by measuring the weight and length of each fish. Afterwards, we dissect the fish in order to extract their reproductive organs and otoliths.
The process begins with the star of our study, the fish! We begin with the collection of fish from markets, fisherman or even try to catch them ourselves. We can then transport the fish back to our lab where we have all the equipment necessary to dissect, collect, and store samples.
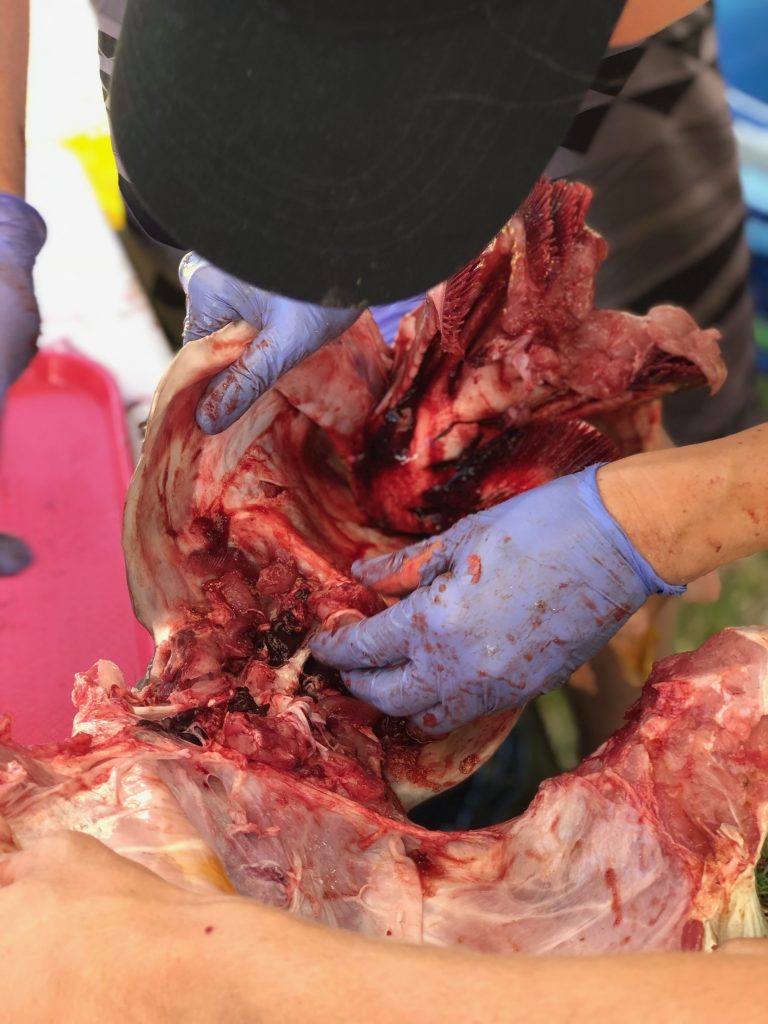
Dissections: Dissecting fish can be tedious and messy at times. This is why we employ specific methods of dissection depending on the morphology of particular species. For example, the gonads in a surgeonfish are located below the stomach and digestive organs, while the gonads in a table boss are positioned above the GI tract. In order to make the dissection easier, we will cut these fish differently.
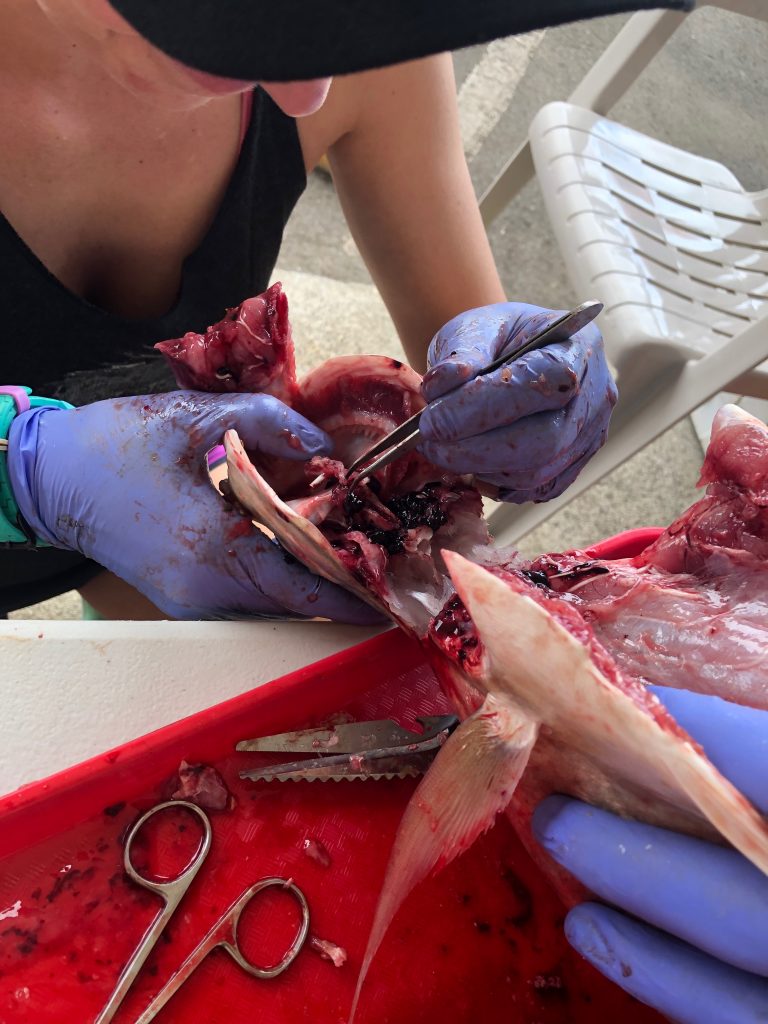
We use two different methods for extracting otoliths, including the “scalp” method which is executed by removing a section of the head just above the eye. This process removes the top of the brain casing giving us access to the brain. We can then remove the the brain and pull out the otoliths which will be sitting in two small pockets beneath it. The second method involves removing the otoliths ventrally and entering the otolith cavity from underneath. Both methods take a degree of practice and patience to perfect.
In surgeonfish the gonads are removed by making a shallow vertical cut through the tissue, extending from the top of the head, down towards the anal fins. The vertebrae is then cut and the head of the fish is pulled down and away from the body. If done right, the internal organs will separate from the body of the fish revealing the intact gonads.
In the typical reef fish, the gonads are located above the gut. Therefore we can begin by making a ventral cut through the fishes’ belly. This is a commonly used practice when gutting fish. Then continue to pull out all of the internal organs, and the gonads will be sitting on top of the gas bladder.
Aging: Otoliths (ear stone or ear bone) consist of three pairs of calcium carbonate structures located in the inner ear of bony fish which aid in hearing, balance, and orientation in the water. Each year, as the fish grows, a new layer is deposited around the otolith. These deposits are the key to determining the age of the fish. By taking a thin cross section of an otolith through the origin and viewing it under a microscope (pictured right), you are able to see alternating dark and light bands. Each combined dark and light band marks one annulus, or year of growth. This is similar to reading rings on a tree!
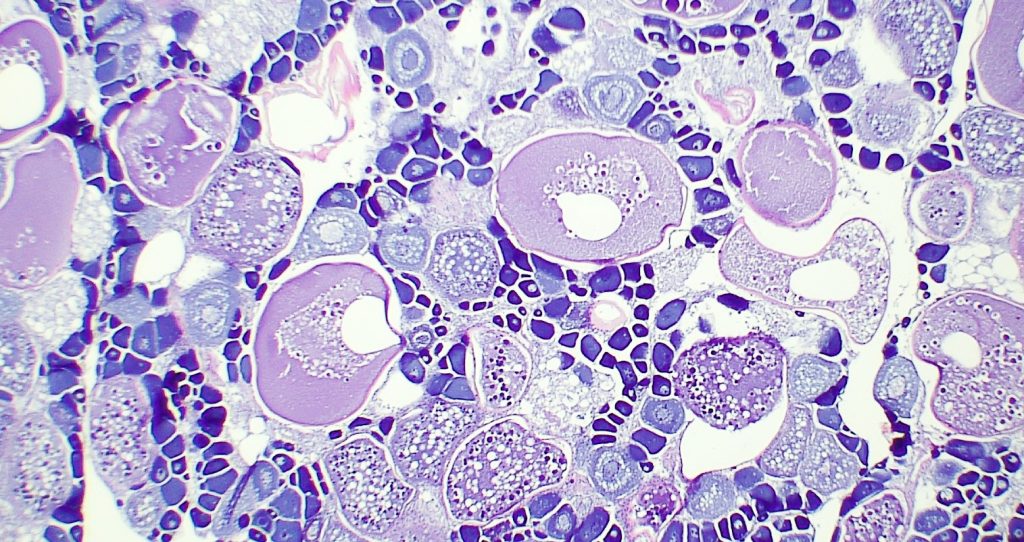
Determining Maturity: The reproductive organs, called gonads, are used in determining the sexual maturity of the individual. First, gonads are removed from the fish and weighed. After the gonads are removed and weighed, a cross section is cut from the middle and placed into a small plastic cassette. The cassettes are stored in 10% formalin, a preservative, to fix the tissues preventing postmortem changes to their structures. The samples sliced into very thin cross sections, attached to a microscope slide, and dyed (pictured right). Each slide is then carefully analyzed to determine sex, maturity, and stage of reproduction based on the cellular structure.